Enroll in a Clinical Study
The National Institutes of Health website ClinicalTrials.gov provides easy and free access to information on clinical studies. Our list below includes all FSHD studies that are currently enrolling volunteers, including some that are not in clinicaltrials.gov. After identifying a trial that you are interested in, the next step is to contact the study coordinator listed on the page and ask for details about enrolling.
In addition to studies listed below, you can volunteer to join a patient registry or make arrangements to donate tissue at surgery or upon death through a tissue donation registry.
Studies recruiting research subjects
The purpose of the study is to evaluate new ways to diagnose neuromuscular diseases. Neuromuscular diseases (NMDs) can affect the nerves that control the muscles you move voluntarily or the muscles themselves. The techniques used in this research are experimental, and have not been approved by the FDA or any other health authority. This research will evaluate the validity of new neuromuscular disease testing, and could identify new neuromuscular disease genes. You will be asked to provide a saliva sample. We will then collect the DNA from the sample (DNA stands for deoxyribonucleic acid. It is the genetic code of organisms). The DNA will be sequenced and checked for any changes or mutations. In addition, the DNA will be checked for epigenetic changes.
If you are interested in learning more, please contact Peter Jones.
Established with funding from the U.S. National Institutes of Health (NIH), the registry is a database of U.S. patients diagnosed with DM or FSHD who are interested in participating in research about these diseases. Their unaffected family members are also invited to join. The National Registry assists researchers looking for volunteers to participate in their studies by searching the registry database for qualified members. The registry staff sends those members a letter announcing the project. Applications are accepted from members and researchers across the United States. To enroll, people are required to complete a comprehensive questionnaire.
If you would like to participate or have questions, please contact: Leann Lewis, MS Health Project Coordinator at the University of Rochester Medical Center/Fields Center/Neuromuscular Disease Center Phone: 585-275-7680 Email: leann_lewis@urmc.rochester.edu The National Registry of Myotonic Dystrophy and FSHD 601 Elmwood Avenue, Box 673 Rochester, NY 14642-8673 USA Toll free: (888) 925-4302 (9 a.m. to 4 p.m. weekdays, EST); Local (Rochester, NY): (585) 276-0004 Fax: (585) 273-1255; Email: dystrophy_registry@urmc.rochester.edu; Web: http://www.dystrophyregistry.org
This study is being run at the FSHD Clinical Trial Research Network sites. To find a site near you that is currently recruiting volunteers, visit clinicaltrials.gov.
Brief Summary: The primary cause of facioscapulohumeral muscular dystrophy (FSHD), a common adult-onset dystrophy, was recently discovered identifying targets for therapy. As multiple drug companies pursue treatments for FSHD, there is an urgent need to define the clinical trial strategies which will hasten drug development, including creating disease-relevant outcome measures and optimizing inclusion criteria. This proposal will develop two new outcome measures and optimize eligibility criteria by testing 160 patients in 7 sites over a period of 18 months. For more information, visit clinicaltrials.gov.
Physicians and researchers at the University of Massachusetts Medical School (UMMS) seek individuals with facioscapulohumeral muscular dystrophy (FSHD) to participate in an FSHD Biomarker Study. This will be conducted by Dr. Robert H. Brown, Jr. and Lawrence J. Hayward, M.D., Ph.D. This study focuses on explaining the variability of FSHD, especially within the same families, through examination of both genetics and other biomarkers.
Purpose: The purpose of this study is to identify and understand genes that may explain why people with FSHD have different amounts of weakness in different muscles (different phenotypes). We also aim to identify biological markers that will enable us to follow and predict disease progression or indicate possible responses to treatment in upcoming FSHD clinical trials.
Participation: Blood, saliva, muscle and/or skin samples from individuals with FSHD, some family members, and population controls are being accepted for this research study. Participants will be asked to complete a brief medical/family history questionnaire. Also, the clinicians will ask for permission to review the medical records of those with FSHD to understand the onset and progression of their disease. The University of Massachusetts Medical School will cover costs of the sample collection for participation, except for travel and housing. We are happy to help to make arrangements for the blood and saliva samples to be collected locally.
Requirements for participation. To become involved, you must:
- Be diagnosed with Facioscapulohumeral muscular dystrophy (FSHD) or be a family member of someone with FSHD
- OR Be a control participant with no family history of FSHD
- Be willing to give a blood sample (approximately 8 teaspoons), or in some cases a saliva sample
- Be willing to consider giving a muscle and/or skin sample
- Be willing to complete questionnaires about your general medical/ family history
UMMS Wellstone Center for FSHD: This study is an integral component of the Senator Paul D. Wellstone Cooperative Research Center for FSHD, sponsored by the National Institutes of Health. The overriding goal of the Center is to develop innovative therapies for FSHD. Research projects are conducted by an exceptional team of collaborative investigators led by Charles P. Emerson, Ph.D. (UMMS), Louis Kunkel, Ph.D. (Children’s Hospital of Boston), and Kathryn Wagner, M.D., Ph.D. (Kennedy Krieger Institute at Johns Hopkins School of Medicine). The Center also provides outreach to academic and industry partners and to patient advocacy groups such as the FSH Society to share research materials and to connect with individuals affected by FSHD. Further information about the Center: https://www.umassmed.edu/wellstone/
Benefits: Although there are no direct benefits for those involved in this research, we believe that understanding FSHD will lead to more effective screening, diagnosis, treatments, and ultimately a cure for this disease. We greatly look forward to speaking with you to answer any questions you may have and to describe this study in more detail.
For more information, please contact: Diane McKenna-Yasek, RN, BSN Neuromuscular Research Coordinator Phone: (508) 856-4697 diane.mckenna-yasek@umassmed.edu
or Catherine Douthwright, PhD Neurology Research Coordinator Phone: (508) 856-6491 catherine.douthwright@umassmed.edu Brown Neuromuscular Laboratory University of Massachusetts Medical School Room S5-710 55 Lake Ave. North Worcester, MA 01655 Fax: (508) 856-4675
Download brochure here.
Your participation is essential to advancing research on FSHD Participants are sought for a University of Minnesota study on muscle stem cells in FSHD.
What is involved? Study participants (FSHD and control individuals) will choose to provide any one or all of the following tissue samples:
- a small muscle biopsy taken with a needle from a muscle in the leg
- skin biopsy
- blood and/or urine sample
What are we trying to find out? What causes FSHD is not well understood. Researchers know that DUX4 dysfunction causes symptoms of FSHD, however they do not know how or why only certain muscles are affected. Researchers have developed a way to study this by looking at muscle in affected and unaffected individuals. We are hopeful that by learning more about what causes FSHD we will be able to develop effective treatments for FSHD.
Will I benefit directly? There is no direct clinical benefit to study participants. This study is aimed at understanding why and how muscle is lost in FSHD. This knowledge is essential to developing a therapy.
How can I participate? This study involves a collection of one or all of the above listed tissue samples from you and a control. For every FSHD participant, we need a corresponding control (unaffected) participant. You can help – if you have a sibling, spouse, loved one, friend, or colleague who would be willing to participate and serve as your control, please do let us know.
Thank you for your support! Isolating and studying cells from controls and individuals with FSHD is critical for advancing our understanding and future treatment of this disease.
*Please contact research assistant Natalya Burlakova at 612-626-4690 or burla019@umn.edu
Approved for use by UMN IRB Effective on 5/30/2018 IRB Study Number: STUDY00000409
The Cardiovascular Research & Rehabilitation Laboratory within the Program of Physical Therapy and Rehabilitation Sciences at the University of Minnesota is conducting a research study to understand if the resting metabolic rate and cardiovascular response to exercise are affected by the genetic mutation that causes for facioscapulohumeral muscular dystrophy.
We are seeking volunteers who have been diagnosed with facioscapulohumeral muscular dystrophy to participate in our study. Volunteers must be over the age of 18 and not be pregnant or nursing. This study requires a one-time visit to the lab which will last one and a half hours.
If you are interested in hearing more about this study, please contact the study coordinator. Contact information is included in this letter as well as on the attached flyer. If you are interested in learning more about our laboratory and our research please visit our website. We appreciate your consideration and look forward to talking with you further.
Contact Information: Study Coordinator: Mia Larson 612-624-6534/crrl@umn.edu
The University of Washington is seeking individuals to participate in a research study aimed at identifying biomarkers of Facioscapulohumeral Dystrophy (FSHD). This study will assess and compare muscle and blood biomarkers in individuals both with and without FSHD. The study is enrolling participants under the direction of Dr. Leo Wang, MD, Neurology Assistant Professor. Blood and muscle tissue samples will be used to better understand FSHD and identify biomarkers of the disease. We are hopeful this research will provide information for development of therapies in the future. All participants must be 18 to 75 years of age and NOT taking any medication for anticoagulation (blood thinners). The study requires one visit lasting about 2.5 hours to the University of Washington Medical Center for the collection of blood and muscle biopsy samples. Participants will be given a stipend of $200. If you are interested and/or would like additional information, please call Susan, the study coordinator at 206-685-2028 or email at rileys@uw.edu. This study is sponsored by Friends of FSH Research.
Participants are sought for a University of Minnesota study on muscle stem cells in FSHD. “We are studying the muscle stem cell in FSHD, and in particular are studying what the DUX4 protein is doing in these stem cells. Although the genetics of FSHD clearly implicate the DUX4 protein in the disease, we do not understand how DUX4 leads to muscle loss. This study addresses this question.
Study participants (FSHD and control individuals) will provide a small muscle biopsy from the quadraceps. The biopsy is taken using a needle and performed by Dr. Karachunski in the Muscular Dystrophy Clinic. Topical anesthetic is used.”
For more information, download the study recruitment flyer.
Genila Bibat, MD, and Kathryn Wagner, MD, PhD Center for Genetic Muscle Disorders, Kennedy Krieger Institute, Baltimore, Maryland Download Kennedy Krieger Study recruitment flyer. The study is especially seeking to increase the number of participants from ethnic minority groups. The Center for Genetic Muscle Disorders at the Kennedy Krieger Institute is a member of the US National Institutes of Health (NIH) Wellstone Muscular Dystrophy Cooperative Research Center, “Biomarkers for Therapy of FSHD.” This group includes basic science, clinical, and translational researchers at multiple academic institutions coordinated through the University of Massachusetts Medical School. The Center is actively involved in several research studies focused on discovering the mechanisms of disease and developing improved methods of studying muscle in FSHD. One of their most successful efforts has been the muscle biopsy program, which was designed to systematically collect and characterize muscle tissue samples that could be shared among multiple FSHD researchers. So far, the muscle biopsy program has succeeded in collecting muscle tissue from more than 80 individuals. These muscle samples are not only being used by researchers in the Wellstone, they are also being transformed into cell lines that can be stored and shared with outside research groups and used in future studies of FSHD. The Wellstone muscle biopsy program is now in the process of expanding to include a second site, the University of Massachusetts Medical School. The muscle biopsy program has also expanded within the Kennedy Krieger Institute to include a non-invasive imaging biomarker project. One of the unique features of the Wellstone study is the co-enrollment of unaffected family members along with individuals who have FSHD. This has proven to be a valuable asset to the Wellstone’s research efforts in that it allows researchers to more accurately judge whether genetic variations seen in a person’s muscle tissue are related to FSHD or if they are normal variations that belong to that person’s family. An unexpected outcome of the FSHD biopsy study was the discovery of several individuals who participated as the unaffected control family member, but were found to have the gene mutation that causes FSHD. Many of these individuals, “non-manifesting carriers,” did not have weakness although they did have the same mutation as their affected family members. A new research project is now under way to determine what factors cause some people with the mutation to have severe disease and others to have normal strength. It is possible that there are major genetic modifiers (including the SMCHD1 gene, which was recently found to be associated with FSHD2, also called FSHD1B, a less common type of FSHD) which do not cause disease by themselves, but have a significant effect on the symptoms of the disease. These genetic modifiers not only would provide new information on the pathologic processes that govern FSHD, but they could also provide new targets for therapies. In order to identify and study these non-manifesting carriers of FSHD, researchers in the Wellstone are working on recruiting larger families of individuals with FSHD. They are particularly interested in recruiting individuals who have a first-degree relative (parent, child, or sibling) with FSHD, but do not have any symptoms of muscle disease themselves. These individuals will be asked to undergo genetic testing for the mutation that causes FSHD in addition to neurological evaluation to confirm the absence of muscle weakness or other signs of FSHD. Those who are found to be non-manifesting carriers of FSHD may be asked to undergo further testing, such as muscle MRI, to assess for subtle signs of disease. This exploration of the milder end of the FSHD spectrum represents a new avenue of research in FSHD, and the investigators of the Wellstone are very hopeful that it will forge new collaborations within the FSHD research community and build on the tremendous progress that has been made in the field of FSHD research in recent years. Individuals or families who would like to receive more information about this study should contact Genila Bibat, the study coordinator for the Center for Genetic Muscle Disorders, at (443) 923-2697 or bibat@kennedykrieger.org.
Sponsor: Centre Hospitalier Universitaire de Nice Information provided by (Responsible Party): Centre Hospitalier Universitaire de Nice
Brief Summary: The investigators propose to conduct a comparative pilot cognitive and psychiatric profiles of 10 patients Facio-Scapulo-Humeral Dystrophy (= FHSD) type 1 and 10 patients with type 2 FSHD study. For this, the investigators relied on observational components: FSHD2 patients appear more often present with psychiatric comorbidities and seem to have lower cognitive performance compared to FSHD1 patients. This was confirmed by a preliminary study on a small sample population of patients. It seems to exist mainly executive dysfunction associated with attention disorders in patients FSHD2. Moreover, their performance in IQ tests would be low in relation to their socio-educational and compared with patients FSHD1 level.
Locations: Hôpital Pasteur, Nice, France, 06002 Contact: Muriel LAFFON, Dr 04 92 03 82 69 ext +33 laffon.m@chu-nice.fr Principal Investigator: Muriel LAFFON, Dr Sponsors and Collaborators Centre Hospitalier Universitaire de Nice.
For details visit https://clinicaltrials.gov/ct2/show/NCT02032979
Sponsor: Murdoch Childrens Research Institute Information provided by (Responsible Party): Murdoch Childrens Research Institute
Brief Summary: This multi-centre, randomised, double-blind, placebo-controlled crossover trial will compare changes in strength-related motor function following treatment with creatine monohydrate to treatment with placebo, as measured by the Motor Function Measure, from baseline to 12 weeks. Eligible subjects will undergo baseline assessments then will be randomised to either creatine monohydrate therapy or placebo for three months, followed by a six week wash-out period, then crossover to a further three months of therapy with either placebo or creatine. Subjects will undergo clinical assessments and study safety assessments at the beginning and end of each treatment period. The study will begin recruitment in early 2017. For further details visit https://clinicaltrials.gov/ct2/show/NCT02948244
Sponsor: Boston Children’s Hospital Collaborator: National Institute of Neurological Disorders and Stroke (NINDS) Information provided by (Responsible Party): Louis Kunkel, Boston Children’s Hospital
Brief Summary: The purpose of this study is to identify genes and proteins responsible for nerve and muscle disorders by studying genetic material from individuals with neuromuscular disease, as well as their family members. We are interested in recruiting many types of neuromuscular disease including; Duchenne muscular dystrophy (DMD), Becker muscular dystrophy (BMD), and limb-girdle muscle dystrophy (LGMD). There are still many patients diagnosed with muscular dystrophy but have no causative gene implicated in their disease. We feel that these patients may have new genetic changes in genes coding for important muscle proteins that we have yet to identify. Using molecular genetics to unravel the biochemical basis of these neuromuscular disorders should lead to more accurate diagnosis of these disorders and should lead to potential therapies.
For further details visit https://clinicaltrials.gov/ct2/show/NCT00390104
Studies not currently recruiting
This study is a Phase 2, randomized, double-blind, placebo-controlled, parallel-group, multicenter study designed to evaluate the efficacy and safety of losmapimod in treating patients with Facioscapulohumeral Muscular Dystrophy (FSHD) over 24 weeks. Patients will participate in this study for approximately 29 weeks. This will include a 4-week screening period, a 24-week, placebo-controlled treatment period and a 7 day safety follow-up period.
Patients must have a confirmed diagnosis of FSHD1 and genetic confirmation must be obtained prior to the screening MRI and baseline muscle biopsy. Patients will be randomized to receive 15 mg of losmapimod or placebo tablets by mouth for 24 weeks. All patients will be asked to attend the study clinic for each scheduled visit. The primary endpoint of the study is to evaluate the efficacy of losmapimod in inhibiting or reducing expression of DUX4, as measured by a subset of DUX4-regulated gene transcripts in skeletal muscle biopsies of FSHD patients. Secondary endpoints include evaluation of the safety and tolerability of losmapimod, the plasma concentrations of losmapimod, levels of losmapimod in skeletal muscle and losmapimod target engagement in blood and skeletal muscle in FSHD patients. For more details and updates on trial sites, please visit: https://clinicaltrials.gov/ct2/show/NCT04003974.
UPDATE: Acceleron has halted development of ACE-083 for FSHD following its Phase 2 trial findings.
Cambridge, Massachusetts-based Acceleron Pharma, Inc., is conducting a study of adults with FSH muscular dystrophy for a Phase 2 clinical trial of ACE-083.
ACE‐083 is an investigational drug that inhibits selected proteins in the transforming growth factor‐beta (TGF‐β) superfamily involved in the regulation of muscle size and strength. ACE‐083 has been designed to increase muscle size and strength specifically in the muscles into which the drug is administered. Acceleron is developing ACE‐083 for diseases in which improved muscle strength in a specific set of muscles may provide a clinical benefit to patients, such as FSHD. As an “investigational” agent, ACE‐083 is not approved by any regulatory agency for use in any country. For full details of the clinical trial, including updates on new clinical trial locations, please visit ClinicalTrials.gov: Study of ACE-083 in Patients With Facioscapulohumeral Muscular Dystrophy (FSHD).
Additional details can be downloaded here:
This research study is being done to study changes in muscle imaging over time in people with facioscapulohumeral muscular dystrophy (FSHD). Whole-body magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) will be used to evaluate skeletal muscle in study participants. This research is being done to assess how changes in muscle imaging correspond to muscle strength and function. Qualified participants will be asked to complete 5 study visits over 21 months. Each visit will include muscle strength and function testing in addition to the MRI/MRS scan. The investigators plan to use MRI and MRS in developing outcome measures that can be used in future clinical trials for FSHD.
This study is seeking volunteers age 12 and older with a confirmed genetic diagnosis of FSHD. If interested, contact: Doris G Leung, MD, PhD 443-923-9521 leungd@kennedykrieger.org
The Johns Hopkins Hospital and the Kennedy Krieger Institute are looking for near relatives of FSHD patients ages 35 and older who do not currently show symptoms. Volunteers will be asked to give a blood draw, which can be performed at any local lab. The blood draw, the genetic test, and shipping will be covered by the study.
Interested individuals should contact Pegah Dehghan: dehghan@kennedykrieger.org.
Study Protocol Number: NA-00019985. Download study flyer.
Sponsor: University Hospital, Montpellier
Brief Summary: Facioscapulohumeral muscular dystrophy (FSHD) is an autosomal dominant disease characterized by progressive weakness and atrophy of specific skeletal muscles. One of the major problems of patients affected by FSHD is the limitation in performing daily activities induced by the progressive muscle weakness. This sedentary lifestyle can cause a “debilitative cycle,” and neuromuscular deconditioning can even aggravate the muscular deficiencies. Recent studies have indicated the safety and the effectiveness of moderate aerobic training programs in patients with FSHD. However, these training programs have limited applicability in patients with more severe muscular weakness. Artificial strength training by means of neuromuscular electrical stimulation (NMES) appears to be a promising rehabilitation strategy for FSHD patients suffering from neuromuscular disorders. Therefore we propose to investigate the feasibility, safety, and effectiveness of NMES strength training to counteract quadriceps muscle weakness in patients affected by FSHD.
For further details, visit https://www.clinicaltrials.gov/ct2/show/NCT02861911
Brief Summary: On the basis of published data and our results indicating that oxidative stress may contribute to the peripheral skeletal muscle dysfunction in patients with FSHD, investigators conducted a pilot randomized double-blind placebo controlled trial to test whether oral administration of vitamins and mineral could improve the physical performance of patients with FSHD.The result of this clinical trial showed that antioxidants supplementation may improve skeletal muscle function of patients with FSHD and suggest that an antioxidant strategy adapted may be a relevant therapeutic approach for these patients. Since then, patients with FSHD who attend consultation at Montpellier hospital are systematically supplemented with antioxidants according their own blood tests.
For further details, visit https://www.clinicaltrials.gov/ct2/show/NCT02622438
Sponsor: King’s College Hospital NHS Trust Collaborators: King’s College London Barts & The London NHS Trust Muscular Dystrophy UK University Hospital Southampton NHS Foundation Trust Information provided by (Responsible Party): King’s College Hospital NHS Trust
Brief Summary: In adults, muscle diseases are usually chronic long-term conditions that do not have a definitive cure. Supportive care has been shown to reduce complications from muscle disease and improved survival in some cases. However, there has been limited research to evaluate interventions that may improve quality of life (QoL) with this patient group. The QoL of those with MD is not just affected by the severity of their MD but also a variety of psychological variables. Based upon the knowledge of these psychological variables the investigators feel that a particular type of psychological intervention known as “acceptance and commitment therapy” (ACT) could potentially improve QoL in those with MD. The investigators therefore propose to test whether ACT does in fact improve QoL in those with MD by randomising 154 patients to receive either standard medical care plus a guided self-help ACT programme, or standard medical care only.
For further details, visit https://clinicaltrials.gov/ct2/show/NCT02810028
Purpose Researchers at the University of Rochester in Rochester, NY, are looking for individuals with FSHD who are interested in participating in a research study to learn more about a potential symptomatic therapy for FSHD. This study may help determine if a combination of drugs (recombinant human growth hormone [rHGH] and testosterone) can be safely given to patients with FSHD and possibly improve walking, strength, muscle mass, quality of life, and functional ability.
What’s involved? The study involves five visits (one with an overnight stay) at the University of Rochester. Travel costs may be reimbursed up to $500 per visit. Study procedures include taking the study drugs (testosterone and rHGH), a physical exam, collection of blood samples, muscle strength and function testing, questionnaires, EKGs, DEXA scans. There is no cost to you to participate, and you will be provided an honorarium after completing four of the visits.
You are eligible to participate if:
- You are a man with FSHD
- You are 18-65 years old
- You are able to walk.
Download the Rochester Study Recruitment Flyer.
For more information, please contact: Liz Luebbe Study Coordinator University of Rochester Medical Center Phone: 585-275-7867 Email: elizabeth_luebbe@urmc.rochester.edu
Rationale According to the NIH Research Portfolio website, the rationale for this study is described as follows:
“Large scale clinical trials have found that testosterone combined with recombinant human growth hormone (rHGH) (combination therapy) is well tolerated and effective in synergistically improving respiratory function, lean body mass, protein synthesis, strength, and aerobic endurance in healthy adult human populations. Both testosterone and rHGH are readily available and approved for human use but have never been formally studied together in a muscular dystrophy population. We propose a 36-week, proof-of-concept clinical study of the safety and tolerability of daily rHGH combined with biweekly testosterone injections in men with FSHD. All participants will be serially and closely monitored during a 24 week period of combination therapy followed by a 12 week washout period. Safety assessments will include monitoring for medication side effects, laboratory abnormalities, physical exam changes, and EKG alterations. As a secondary objective, we will examine the pharmacokinetic effects of combination therapy on lean body mass and serum biomarkers. Participants will also have serial assessments of their ambulation, strength, physical function, patient-reported disease burden, and respiratory function. Ultimately, this study will generate extensive data regarding the clinical safety, pharmacokinetics, and change in body composition and clinical function associated with combination therapy in a predefined FSHD population.”
Also see the study listing on ClinicalTrials.gov.
Recent advances in our understanding of FSHD have identified for the first time, since discovering the mutation behind FSHD 20 years ago, a potential target for therapy, and the research community has shifted towards clinical trial planning. However, hampering these efforts are the wide variability in disease expression, which at its heart, may be due to the epigenetic nature of the disease.
A barrier to identifying genetic or environmental modifiers of disease has been the lack of a clinically meaningful tool for documenting progression of disease. Such a rationally built scale would be calibrated so each increment in the scale would reflect a progression in the clinical disability of the disease. Such a scale would enable the identification of genetic and environmental modifiers of disease expression, while at the same time providing a powerful tool to stratify patients for future clinical trials, potentially reducing the variability and increasing the likelihood of identifying potentially effective therapeutics. In addition such a tool would be invaluable for prognosis and surveillance in the clinic.
Here we propose to develop a scaled and calibrated Rasch-built clinical severity scale for FSHD (the FCSS). We are seeking fifty (50) volunteers, twenty-five (25) from the University of Kansas Medical Center, to participate in this study. Volunteers will be required to make a single visit lasting approximately 6 hours. Anyone with a diagnosis of FSHD who can travel to and from the University of Kansas Medical Center is eligible for this study.
Contact: Ayla McCalley, Neuromuscular Research Center, University of Kansas Medical Center, 3901 Rainbow Boulevard, Mailstop 2012, Kansas City, KS 66160. Phone: 913.945.9937. Email: amccalley2@kumc.edu
Download for further details: Rasch-Analysis Study Fact Sheet.
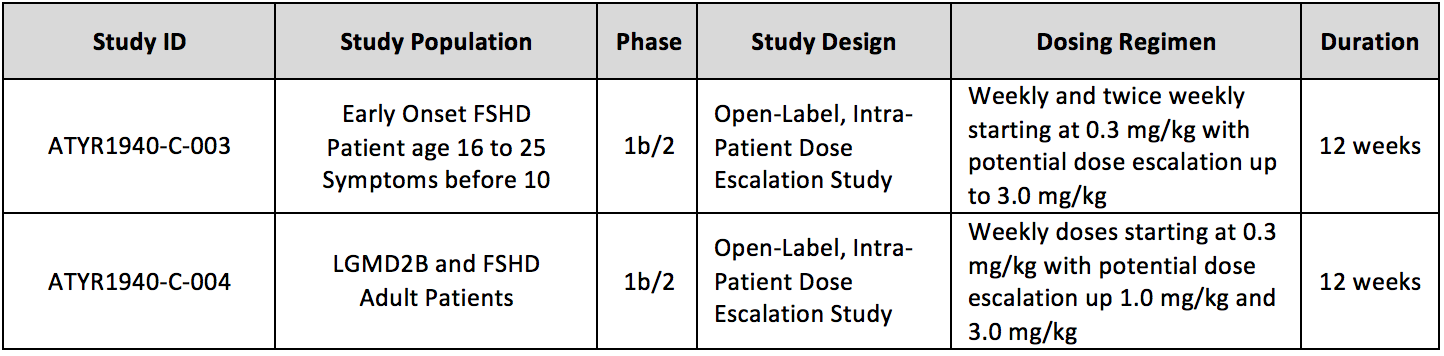
aTyr Pharma, a company engaged in the discovery and development of therapeutics to address severe rare disease, is currently conducting two trials of Resolaris (ATYR1940) in patients with rare myopathies with an immune component:
The trials are designed to assess the safety, tolerability, immunogenicity and activity of Resolaris in patients with the muscular dystrophies of FSHD or limb-girdle muscular dystrophy 2B (LGMD2B or dysferlinopathies). ATYR1940-C-003 is open for enrollment. ATYR1940-C-004 has nearly completed enrollment and is currently closed to screening.
aTyr Pharma recently announced results from its first Phase 1b/2 trial in adult patients with FSHD. To review the press release, please visit the Press section at www.atyrpharma.com.
What is Resolaris (ATYR1940)? aTyr Pharma is developing Resolaris as a potential protein therapeutic for patients with severe, rare mypotathies with an immune component, for which there are limited or no approved treatments. Resolaris is derived from a naturally occurring protein released in vitro by human skeletal muscle cells The Company believes Resolaris could potentially play a role in promoting skeletal muscle health by acting as an immunomodulator in skeletal muscle. Inflammation is believed to play a role in the disease process in FSHD and LGMD2B. For additional information, please visit www.atyrpharma.com.
Where are the trials conducted? Clinical sites for both studies are located in the United States and Europe as set forth below.
ATYR1940-C-003 Study:
- Stanford University, Palo Alto, California
- University of Iowa Children’s Hospital, Iowa City, Iowa
- University of Utah, Salt Lake City, Utah
- Institut de Myologie, Paris France
- Fondazione I.R.C.C.S. Istituto Neurologico Carlo Besta, Milano Italy
ATYR1940-C-004 Study:
- University of California, Irvine, ALS and Neuromuscular Center, Irvine, California
- Kennedy Krieger Institute, The Johns Hopkins University School of Medicine, Baltimore, Maryland
- OSU Wexner Medical Center, Columbus, Ohio
- Rigshospitalet, University of Copenhagen, Copenhagen, Denmark
- Institut de Myologie, Paris France
- Fondazione I.R.C.C.S. Istituto Neurologico Carlo Besta, Milano Italy
Where can I find more information on these trials? Please visit the following websites to get more detailed information: www.clinicaltrials.gov (type ATYR1940 in “Search studies”) and www.atyrpharma.com. You can also contact aTyr Pharma by email at clinicaltrials@atyrpharma.com or phone at 1-877-215-5731.
Can I participate in one of these trials? To be enrolled in the ATYR1940-C-003 study, you should be between 16 and 25 years old, have a genetic confirmation of FSHD and have had onset of FSHD symptoms prior to 10 years of age. You will need to be seen at a participating center. Some history of other diseases or some medication taken can prevent you from participating in the studies. Please talk to your doctor about your individual disease status and whether you might be suitable for one of the studies.
Download the aTyr trial information.
A Placebo-Controlled, Randomized, Multiple Ascending Dose Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Biological Activity of ATYR1940 in Adult Patients With Molecularly Defined Genetic Muscular Dystrophies. For clinical trial eligibility criteria and locations, visit clinicaltrials.gov. May 2015 presentation on Resolaris here.
Patients in the Southern California region have an opportunity to participate in a clinical study of FSHD. The study is sponsored by aTyr, a biotechnology company in San Diego, in collaboration with Sanguine, a company in Sherman Oaks, California, that collects biospecimens directly from patients in their homes on behalf of clients that include companies, research institutes and patient advocacy organizations. Sanguine will be compensating donors for the sample collections ($50 each). Dr. Melissa Ashlock, Vice President, External Scientific Alliances & Human Genetics at aTyr Pharma, explains the purpose of the aTyr study. If you have any questions, please contact June Kinoshita at june.kinoshita@fshdsociety.org or 781-301-6649.
Lauren Hache, Clinical Research Manager, Cooperative International Neuromuscular Research Group (CINRG) Children’s National Medical Center, Washington DC The Cooperative International Neuromuscular Research Group (CINRG) site at the Children’s National Medical Center in Washington DC is recruiting patients with infantile-onset FSHD. To date, we have identified 30 eligible participants from participating CINRG sites globally; we look forward to further enrollment and data collection over the coming months. During a third year no-cost extension Drs. Mah and Yi-Wen Chen are designing new research projects based on this growing cohort of individuals with a relatively rare but more severe form of FSHD and are moving forward with data analysis as well as collecting further samples for expression-profiling studies to be done at Dr. Chen’s lab. Study Name: A multicenter collaborative study of the clinical features, expression profiling, and quality of life of infantile onset FSHD. Study Overview: This study is being run by the Cooperative International Neuromuscular Research Group (CINRG). More information about CINRG and its research studies can be found at the CINRG website. This study is an observational study that aims to advance our knowledge of infantile-onset FSHD. The study will include 50 participants of all ages who have presented with symptoms of FSHD between birth and 10 years of age. Study participation will involve a single day of assessments at one of the participating CINRG centers (to include physical exam, cognitive testing, eye exam, hearing test, strength testing and speech evaluation). The procedures may be split over additional days for scheduling purposes. Inclusion Criteria: Clinical diagnosis of FSHD including the presence of all of the following features based on review of medical records and/or direct examination: 1) onset of symptoms involving the facial or shoulder girdle muscles; and 2) genetic confirmation (according to the study defined criteria for FSHD). How to Become Involved: For more information about the study and a list of participating CINRG sites please view ClinicalTrials.gov or the CINRG website. The FSH Society is providing stipends for patients who cannot afford the expense of traveling to one of the study sites. Travel costs will be reimbursed after you have completed participation in the study and submitted travel receipts. For more information about enrollling in the study please contact the Lauren Hache, MS, CGC, Operations Manager, at (412) 224-2030 or LHache@childrensnational.org to determine if you are eligible and to be referred to a participating CINRG site.
Purpose of Study – To investigate a new method to measure functional mobility in FSHD population in preparation for future therapeutic clinical trials
About the Study
- Up to 2 visits to the Acute Rehab Unit or outpatient PT/OT clinic at University of California, Irvine Medical Center in Orange, CA
- Up to 1 hour for each visit
- $200 compensation after completion of the study
- No cost to you or insurance
- Lead Researcher of this study: Jay Han, M.D., UC Irvine, Department of Physical Medicine and Rehabilitation
If interested, please contact: Vicky Chan, DPT Phone: 949-447-8339 Email: vchan2@uci.edu And ask about the “The FSHD Study”
Download flyer here.