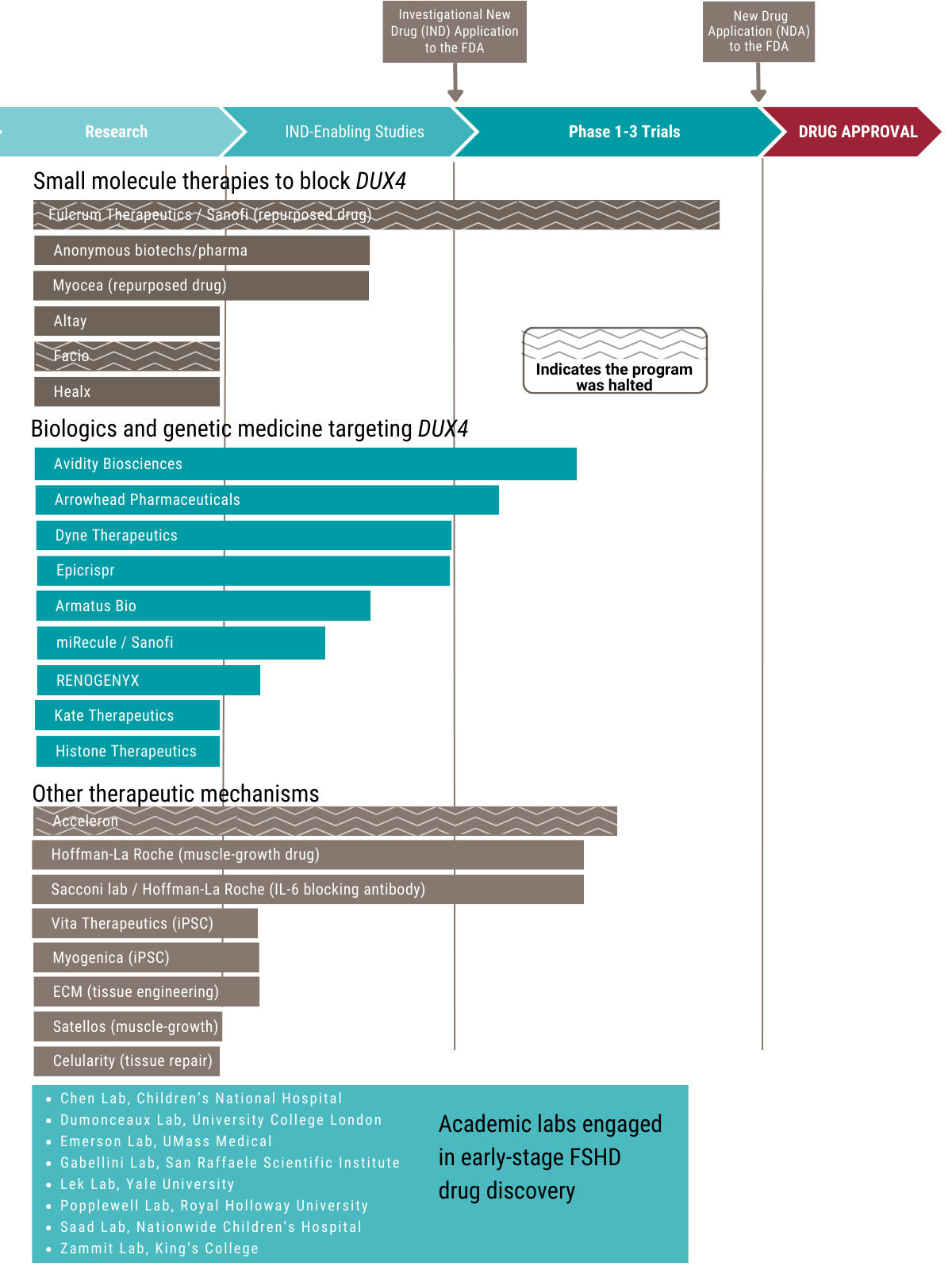
Drug Development Pipeline
A Growing Number of Treatments in Development for FSHD
More and more companies and research labs are working on early-stage treatments for FSHD. These efforts are part of what’s called the drug development pipeline—the process of finding and testing new medicines.
Types of drugs being developed
Many of the drugs in development for FSHD are designed to block a gene called DUX4, which is believed to be the main cause of FSHD types 1 and 2.
Other drugs focus on helping the body in different ways, such as:
- Calming down the immune system
- Helping muscles grow and heal
Most drugs fall into one of three main types:
- Small Molecules are tiny chemical compounds made in a lab. Because they are so small, they can often be taken as a pill and travel through the body to reach the cells where they are needed.
- Biologics are made from living cells or natural materials like proteins or DNA. They are usually much larger and more complex than small molecules and are often given by injection or through an IV.
- Gene Therapy aims to fix or control a disease by changing how a person’s genes work. This can be done by adding a healthy gene, turning off a faulty gene, or changing how a gene is expressed. Gene therapies are also given through an IV.
*Other possible treatments—like supplements or physical therapy—are also being explored, but they are not shown here as part of the drug development pipeline.
What is a repurposed drug?
Some treatments were first made for other diseases. If one of these repurposed drugs shows promise for FSHD, it can often move quickly into clinical trials. This can save time and money because the drug has already been tested for safety.
Who are the anonymous biotechs & pharmas?
Some companies working on FSHD drugs choose to stay private or “in stealth mode” for business reasons. The FSHD Society is in touch with many of these groups and helps support their early research.
When they go public, we’ll share updates—so be sure to:
How drug development works
The drug development process starts by finding a “target”—something in the body that causes disease and can be changed by a treatment. Scientists then look for compounds (possible drugs) that can affect this target. These first tests usually happen in a lab, often in a test tube.
The compounds that work best are picked as “lead” drugs, and scientists work to make them even better—this step is called lead optimization. Next, the lead compound is tested in animals to see if it is safe and might work—this is called the preclinical phase.
If the results look good, the company can ask the FDA for permission to test the drug in people. This is called an Investigational New Drug (IND) application. If approved, the drug goes through several phases of clinical trials in humans. If it passes all the tests, the company can then ask the FDA for approval to sell the drug by filing a New Drug Application (NDA).
Learn more about the drug development process with this video from the FDA.
Interested in learning more about clinical trials for FSHD? Check out our Clinical Trial Education Hub.
