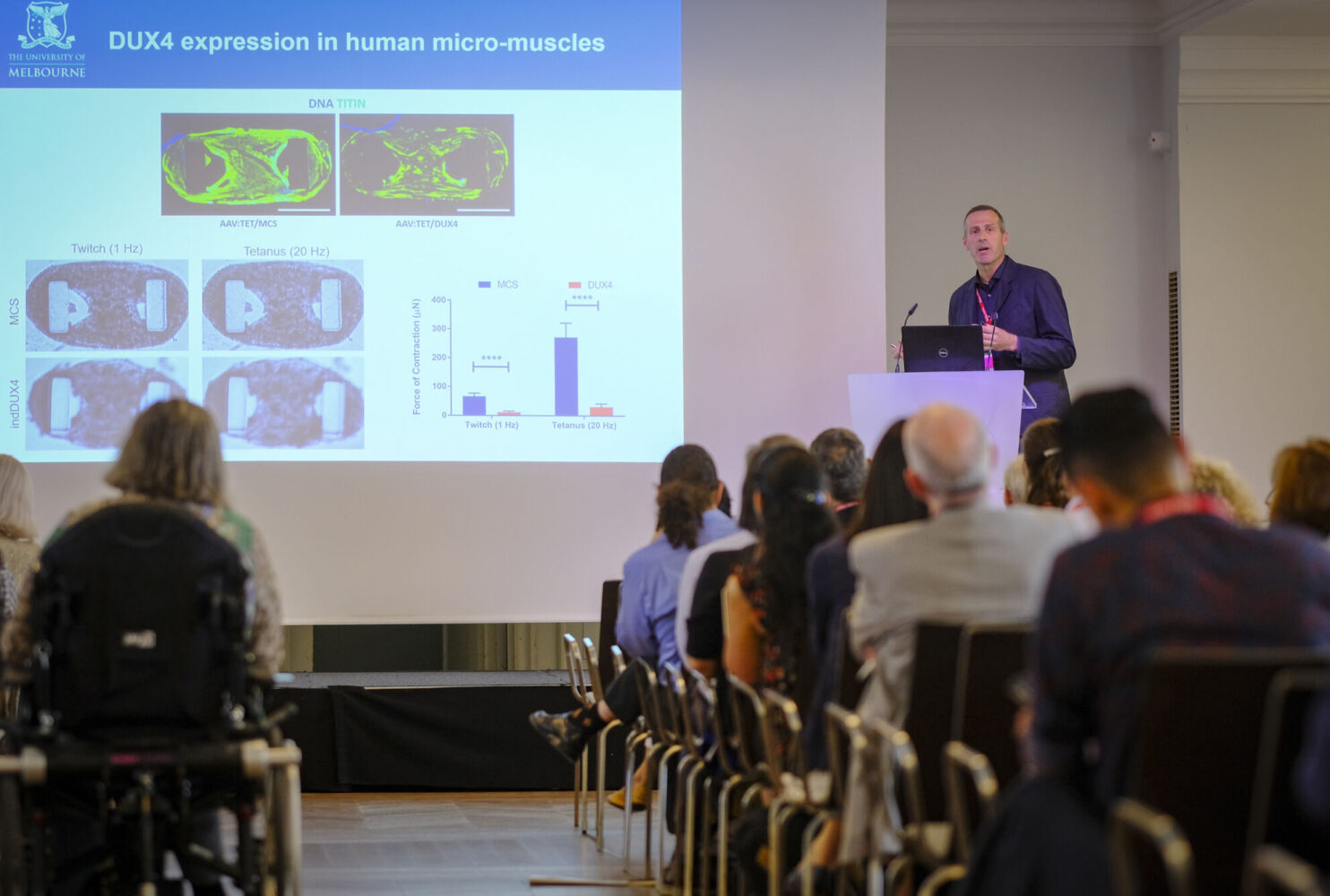
Accelerating Treatments for FSHD
The Global FSHD Innovation Hub is dedicated to speeding up the development of treatments for facioscapulohumeral muscular dystrophy (FSHD) and ensuring patients get access to them as soon as possible. It serves as a one-stop partner for biopharmaceutical companies, providing specialized support throughout the entire drug development process.