Overcoming obstacles to clinical trials and FDA approval to ensure that effective therapies get to families by 2025
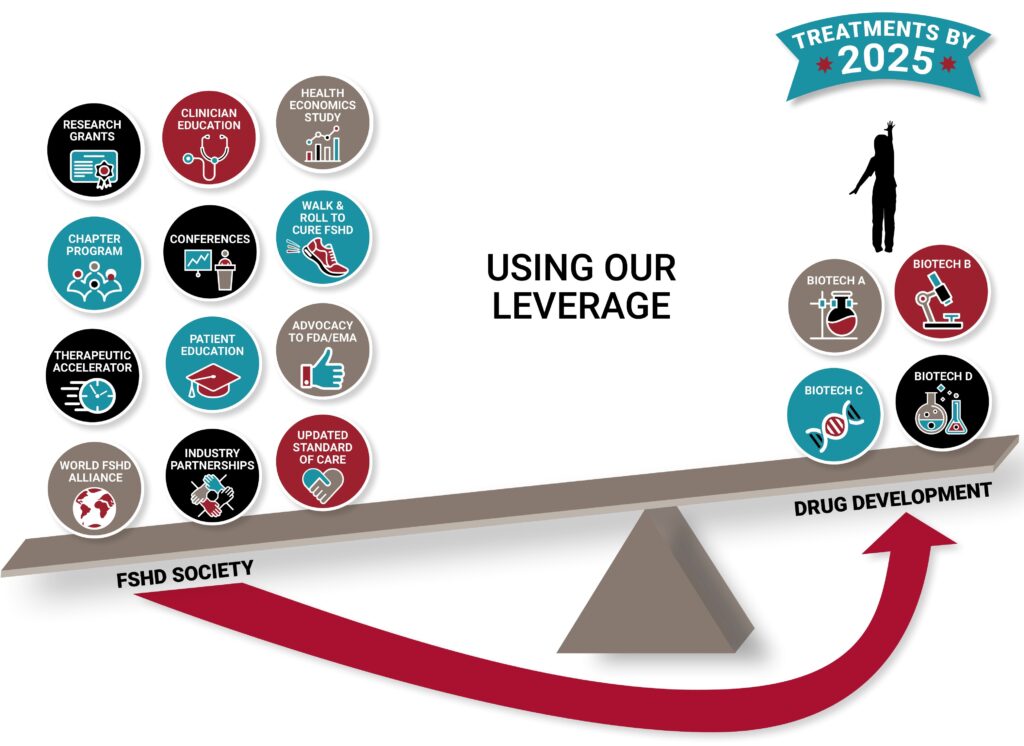
Since the genetic disease mechanism was discovered in 2010, facioscapulohumeral muscular dystrophy (FSHD) research has gained tremendous momentum. Recent breakthroughs have brought us to a new world. It is no longer enough to fund research grants. There is additional essential work to be done to pave the way for successful clinical trials and the approval of effective therapies.
With every affected patient in mind, our Therapeutic Accelerator program seeks to overcome the hurdles and shorten the timeline to get therapies to our families.
More than a dozen companies have drug development programs in FSHD. We listen to their challenges and map out a responsive strategy for overcoming obstacles. Through collaborations and our own initiatives, we are lifting all companies that are developing treatments closer to our shared goal - therapies to families by 2025.
In March 2019, the FSHD Society convened our first Industry Collaborative. This was a landmark meeting attended by FDA regulators as well as researchers from seven biopharmaceutical companies, academic scientists who are leading the effort to prepare the field for clinical trials, and representatives of patient advocacy organizations including the MDA, Friends of FSH, and Chris Carrino Foundation.
The workshop introduced the FDA to FSH muscular dystrophy – the impact of the disease on individuals, why scientists think FSHD is treatable, and the progress that has been made toward clinical trials – and sought input from the FDA on how to develop methods to measure treatment effects to meet the standards for FDA approval.
The findings from the workshop are summarized in a white paper, Industry Collaborative Workshop for Therapy Development in FSHD.
Therapeutic Accelerator Project areas
All of these areas require a coordinated effort to ensure that clinical trial measurement tools meet industry and FDA standards. All drug development companies will have an accepted way to measure the effectiveness of their drugs, and the most promising will go into clinical trials faster.
Genetic diagnosis. Our TestFSHD initiative aims to improve individuals' access to faster, more cost-effective genetic testing. This project fills a significant medical need and will enlarge the number of genetically confirmed patients for clinical trials.
Circulating biomarkers. We strive to develop a blood test that biopharmas can rely on to measure the effectiveness of their drugs in clinical trials that are less invasive than a muscle biopsy.
Imaging biomarkers. While promising as a way to measure whether a drug is working, magnetic resonance imaging (MRI) findings must be confirmed and proven to correlate with other measures such as strength and function.
Integrating global patient registries & natural history studies. We are working on ways to aggregate and analyze data across multiple registries to gain a more rigorous, comprehensive view of the condition.
Patient-focused Drug Development meeting. In June of 2020, the FSHD Society convened the landmark externally-led patient-focused drug development (EL-PFDD) meeting on FSHD. The resulting Voice of the Patient Report has been submitted to the FDA and EMA.
Health economics outcomes research. The Society is analyzing large medical claims datasets and conducting the True Cost of FSHD survey to better understand FSHD prevalence, patient journey, and the direct and indirect economic costs associated with this diagnosis.
International care standards. Updating care standards for the international community will benefit patients and standardize baselines in clinical research and trials.
What we do and why...
Activate Research
In addition to funding record amounts of grant funding, we support the Clinical Trial Research Network (CTRN), foster collaboration, and run strategic initiatives that speed up the development of treatments.
Activate Clinicians
With programs like our Continuing Medical Education (CME) course, we work to speed up the diagnosis and improve the standard of care for patients everywhere, which helps to ensure higher-quality clinical trials.
Activate Community
Programs like our Chapters, FSHD University, Gathering Place groups, and more help cultivate an educated, empowered, and active community of patients and family members, which is critical to speeding up therapy development.
Activate Advocacy
Our Voice of the Patient Report, Health Economics Outcomes Research (HEOR), and other initiatives ensure that regulatory agencies and payors understand the real-life impact and urgency of getting treatments to patients.
Interested in learning more about this exciting project?
- Contact: June Kinoshita
Senior Director of Research & Education, FSHD Society
To pledge your support
- Contact: Mark Stone
Chief Executive Officer, FSHD Society

Anybody can do the
ordinary…We are being
challenged to do the
extraordinary!
- Mark Stone
President and CEO


