Proof-of-principle study is the first to use CRISPR technology on the “repeat genome,” as well as its first successful use in primary human muscle cells
BOSTON – (November 3, 2015) – The FSH Society, the award-winning non-profit and global leader in the quest to cure Facioscapulohumeral Muscular Dystrophy (FSHD), announced that an FSH Society-funded research team led by Peter Jones, PhD, at the University of Massachusetts Medical School (UMMS) has successfully used a derivation of the CRISPR-based gene-editing method known as dCas9 to target and silence the DNA sequence implicated in FSHD. This genetic condition, which affects an estimated 1 in 8,000 people, is among the most common forms of muscular dystrophy.
The work represents two firsts. “While CRISPR technology has been used successfully in early studies of genome editing, this is the first report in which a CRISPR-based system has been used to ameliorate pathogenic gene expression in FSHD,” writes the paper’s lead author Charis Himeda, PhD. “This is also, to our knowledge, the first time the technique has been used successfully in primary human muscle cells.”
The CRISPR/Cas9 system originated from the discovery of a mechanism that bacteria employ to purge their genomes of foreign genes, somewhat like a primal immune system. Molecular biologists have figured out how to harness this natural system to specifically target genomic sequences.
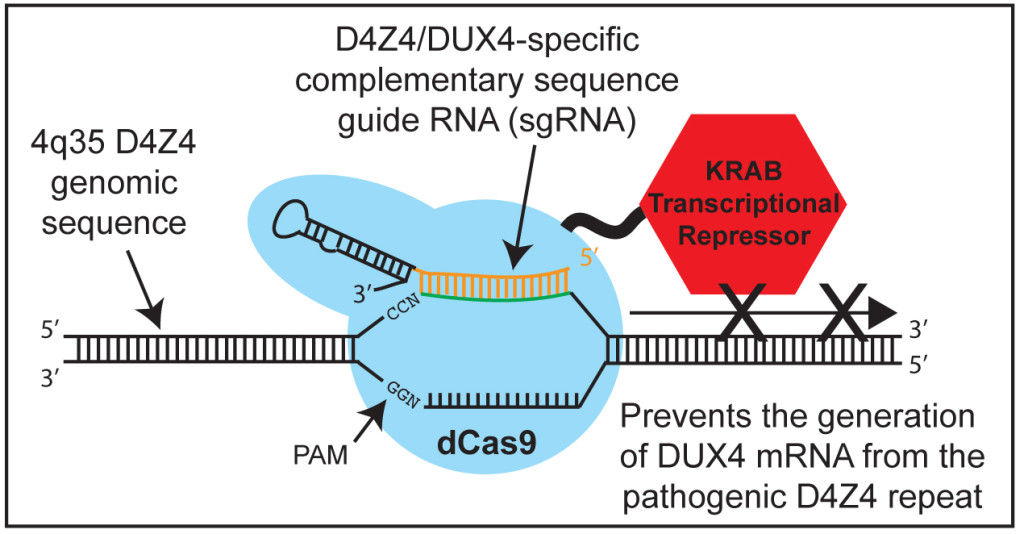
Typically, the CRISPR/Cas9 technology is used to cut the DNA to change or remove specific sequences. However, the potential off-target effects of introducing non-specific cuts to the genome are a serious concern. As an alternative, the CRISPR/dCas9 system does not cut the DNA, instead altering the expression status of the targeted gene by recruiting either gene activation or repression proteins. In theory, CRISPR/Cas9 could be used to treat classic genetic disorders by editing gene sequences while CRISPR/dCas9 could be used to silence mutant disease-causing genes or activate beneficial genes.
In FSHD, muscle degeneration results not from a misspelled gene but rather from a different type of genetic error. The most common form of the condition, FSHD1, is caused by a shortening of a variable tandem repeat region of so-called “junk” DNA on chromosome 4. This repeat genome region consists of numerous repetitive units called “D4Z4.” Normally, humans have between 11 to over 100 D4Z4 units in this location, but in individuals with FSHD1, there are only between one and 10 units.
This repeat region harbors a gene called DUX4. Normally, this gene is repressed. But in FSHD, the reduced number of repeats, together with loss of methyl groups in the region, causes changes in the structure of the chromatin (the complex of molecules that form the chromosome). The result is that DUX4, and possibly a number of noncoding RNAs, become prone to being expressed, triggering chemical events that lead to muscle destruction.
Several research groups, including ones funded by FSH Society grants, are using CRISPR/Cas9 to edit the DUX4 gene in an effort to render it non-functional. The UMMS group, however, decided to more broadly target several regions of the D4Z4 repeats. “The D4Z4 repeats encode multiple coding and noncoding RNAs, which have the potential to play downstream pathogenic roles in FSHD. Thus, targeting the FSHD locus to return the chromatin to its non-pathogenic, more repressed state might be more therapeutically beneficial than simply targeting DUX4,” the authors explain. The methods developed and demonstrated by this study “should pave the way for more effective and stable correction of FSHD and other epigenetic diseases.”
The work may have powerful implications beyond the relatively rare incidence of FSHD. “With increasing evidence that the repeat genome (comprising nearly half the human genome) plays important roles in gene regulation, additional diseases will likely be found associated with aberrant repetitive genomic sequences,” the authors said. “We have provided the first evidence that the repeat genome can be targeted via the CRISPR system, which is likely to prove useful as this hitherto overlooked portion of the genome is decoded.”
The newly published work was supported by the National Institute of Arthritis, Musculoskeletal, and Skin Diseases grant #1R01AR062587 and the Association Française contre les Myopathies grant #AFM15700. The FSH Society funded work in the Jones lab that laid the foundations for the current study and supported development of the UMMS Wellstone Center FSHD cell and DNA repository used in this research.
Reference
Charis L. Himeda, Takako I. Jones, and Peter L. Jones. CRISPR/dCas9-mediated transcriptional inhibition ameliorates the epigenetic dysregulation at D4Z4 and represses DUX4-fl in FSH muscular dystrophy. Molecular Therapy, accepted article preview online 03 November 2015; doi:10.1038/mt.2015.200. View on PubMed.
See press coverage of this story:


Thank you so much for such good news !
If you agree, I will publish a summary / translation in french of your article and use your drawing on my blog.
Hi Sylvie, please feel free to post a translation. Thanks! – June
Hi,
great news,
Is this treatment availble to persons wanting to get it or is treatment still years away from being available
We are still years from having a treatment based on this technology. But there is progress on other fronts as well. The good news is that we are seeing many different strategies for treating FSHD.
They are already using this technology in treating cancer patients so why slow to do for FSH?
Done !
http://groupefsh.blogs.afm-telethon.fr/archive/2015/11/05/la-technique-d-interference-genique-utilisee-dans-la-myopath-94238.html
So what is next with regard to this technology? Does it sit on the shelf? Is it just another published white paper? What are the steps to develop an available treatment based on this technology? Is there a plan to see these steps through? Is there an effort to find an industry partner to continue development through clinical trials?
Hi please send more information about my son
Have DMD thanks so much for you help
Thank you so much for this publication.
I’m a recently graduated medical translator, so if in the future, you need any translation work done, feel free to pas it on to me ………I’ll be happy to translate…….Sylvie has already done that for this article but I’ll be happy to do the same in the future for different publications
Looking forward to working with you,
Marie Gotteland
Fantasti news!! But how Can have this news Also in Italy?? Here the research about Fsh si really underestimated!!!!! Thanks a lot to American researchers!!!
Is this treatment available to persons wanting to get it or is treatment still years away from being available ?
I am so happy about hearing such good news. I know there is a lot of science and work to do till starting this treatment. But you all have done great work. I’m sure this will lead to an efficient treatment for FSHD. Never give up stopping this horrible disease.
This is remarkable. Is there anything we can do to speed the process? Do you need any human test subjects?
This sounds promising, but my concern is the government stopping this from becoming available as I am already reading articles that are voicing concerns of ethical use. Though I belive in making sure this is a safe answer for this awful disease I also believe that our government is more concerned about dollars and cents when it comes to pharma and “r and d” rather than the lives of the sick. Well I guess we will see what happens.
Please continue to progress our hurry sickness . How Many Years Will Continue this work ?
Can this type of editing help treat people with FSHD2?
We CAN’T wait anymore. We need this treatment NOW!
Can someone provide an update on the progress that is made over last 30 months after the paper was published?