With a little zap, transplanted human cells flourish in a mouse
By June Kinoshita, FSH Society
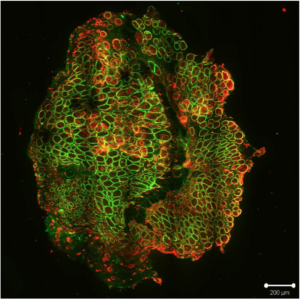
It’s like something out of Dr. Frankenstein’s underground laboratory, but a University of Maryland Medical School team led by Robert Bloch, PhD, has successfully coaxed human muscle precursor cells to grow and form mature muscle in a mouse. Among the tricks used to accomplish this feat was the application of electric currents to the transplanted cells.
The study, published in the journal Skeletal Muscle, notes, “These studies were supported initially by a fellowship … from the FSH Society.”
The researchers used “immortalized myoblasts,” which are immature muscle cells that had been genetically reprogrammed to divide without limit. The cells were provided by Dr. Woodring Wright, an NIH Wellstone Center researcher, who derived them from a muscle biopsy from an FSHD patient and healthy family members. That tissue donation was made possible by FSH Society travel funds that enabled the donors to be seen, evaluated, and biopsied by Kathryn Wagner, MD, PhD, at the Kennedy Krieger Institute in Baltimore, Maryland.
Previously, Wagner’s team had reported on a successful effort to graft slivers of human muscle tissue onto the legs of living mice. (See FSH Watch, Spring 2014.) These “xenografts” provided a way to study human FSHD muscle tissue in an experimental animal. While impressive, this approach was limited by the scarce supply of FSHD muscle and the delicate, time-consuming microsurgery required.
The Bloch lab used immortalized myoblasts, rather than mature muscle tissue, and transplanted the cells into the hind limbs of mice, where the native muscle and satellite (muscle stem) cells had been ablated by radiation and cardiotoxin. This procedure provided a niche for the transplanted myoblasts.
In the first attempts, some of the transplanted cells survived, but they were few and stunted. But then, borrowing from research showing that electrical stimulation helps regenerate injured muscles in human patients, the Bloch lab tried applying pulses of electricity to the peroneal nerve of the limb and found that the transplanted cells survived and grew, so that most of the cells in the newly grown muscle were of human origin.
In the accompanying interview, Bloch answers FSH Watch’s questions about what was especially noteworthy about this research and how it will be applied.
Reference
Sakellariou P, O’Neill A, Mueller AL, Stadler G, Wright WE, Roche JA, Bloch RJ. Neuromuscular electrical stimulation promotes development in mice of mature human muscle from immortalized human myoblasts. Skeletal Muscle 2016;6:4. (PubMed)
Interview with Robert Bloch, PhD, University of Maryland Medical School, Baltimore, Maryland.
Q: What do we know now that we didn’t know before this study?
BLOCH: Before we started our research, no laboratory had successfully generated mature human muscle tissue in mice starting with myogenic precursor cells, or myoblasts. The advantage of using these cells is that they can be grown in very large numbers, and so, if methods allowed, many thousands of mice could be prepared that carried human muscle tissue prepared from an individual, or a set of individuals, with particular characteristics. This is especially important in studies of FSHD, because the disease cannot be fully replicated in mice by genetic methods.
When we started, the best any laboratory had done was create muscles in mice with fibers that were about one-third human in origin, with the rest being from the host mouse—far from the goal of 100 percent human. Our experiments have shown that we can generate human muscles in mice with fibers that are greater than 98 percent human in origin, and that we can use our methods with cells from individuals with FSHD as well as from healthy donors.
We have learned how to make human muscles, including muscles from FSHD patients, grow to maturity in mice.
Q: What does your study mean for patients?
BLOCH: Translating basic science to the clinic requires preclinical experiments to show that a promising treatment for FSHD is efficient and safe. Our study shows that we can prepare large numbers of mice carrying human muscle tissue, including FSHD tissue, to test therapies for FSHD for their efficiency and safety before we test them in human patients in Phase 1 clinical trials.
Q: What are your lab’s near-term aims?
BLOCH: In the next year or two, we will continue to improve our methods to optimize them for the production of mice for preclinical testing. We will also use our mice in collaboration with other laboratories to initiate studies of promising therapies for FSHD.
Q: What are the longer-term goals?
BLOCH: Our long-term goals are, first, to make our technology and mice widely available to research laboratories and biotech firms who are testing potential treatments for FSHD, and second, to determine if our methods can be adapted to repair damaged muscles in patients with myopathies, muscular dystrophies, or severe muscle trauma.A microscopic image of muscle grown in a mouse. The green color indicates the presence of human beta-spectrin protein, proving that most of these muscle fibers originated from the transplanted human muscle precursor cells.


Mouse grows a human muscle…
This is very hopeful and exciting news. I am a 58 year old female diagnosed with FSHD in 1994. I live in Minnesota. I’ve donated muscle tissue three times over the years and my unaffected siblings donated two years ago. FSH is usually a slow progressing disease with spurts of acceleration. I am getting to the point where I don’t want to give up using a walker for a wheelchair or scooter, because even without a disease, if you don’t use it, you lose it. I will not allow myself to lose the ability to walk. Thank you so much for the information and thank you, thank you to everyone who continue this research. My husband and I are supporters of the FSH Society and of course, if possible, I’d love to do my part giving more tissue or participate in a clinical trial . I love music. I love to dance. I hope to dance with my husband again and keep on dancing.
Yes, this article was hopeful to me as well. Reading Linda’s comment encouraged me to respond since we somewhat mirror one another. I’m also 58, diagnosed in 1992 and have hopes to hike with my husband again, Although I use a scooter for long distances, my goal is to remain ambulatory in the home as long as possible. PS, Linda if you read this, I live in Arizona and would enjoy being pen pals if we can somehow connect!