Steady improvement seen in data from open-label extension study
Losmapimod, a drug that is currently in a Phase 3 clinical trial for FSH muscular dystrophy, continues to slow or stop the progression of disease in patients who have been taking the drug for up to 96 weeks, according to Fulcrum Therapeutics, the Cambridge, Massachusetts-based company that is developing the therapy. Jenny Shoskes, PharmD, from Fulcrum presented the data in early October at the World Muscle Society Congress in Halifax, Canada.
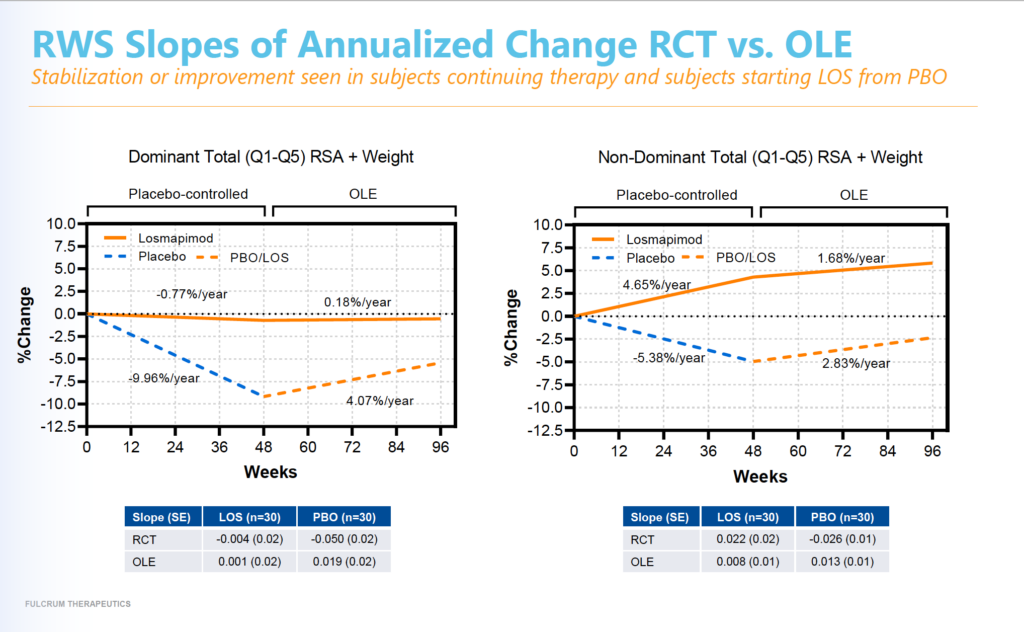
The data were collected from individuals who had participated in the Phase 2 study of the drug that was completed in 2021 and then opted to continue in an “open-label extension” (OLE) study in which all participants were offered losmapimod for an additional 48 weeks.
In the Phase 2 study, called ReDUX4, half of the participants were assigned at random to take 15 mg losmapimod twice daily and half received placebo. The trial was “double blind,” meaning that neither the participants nor researchers conducting the study knew who was receiving drug or placebo. At the end of the initial 48-week study period, all participants were offered the option to join the open-label extension, in which everyone who was on placebo was switched to losmapimod and all who started on losmapimod continued on losmapimod. Of the 77 participants who completed the initial 48-week study, 76 enrolled in the OLE. At week 96, 74 participants remained on treatment.
“Losmapimod can provide meaningful benefits”
In the ReDUX4 trial, individuals who had been on placebo experienced disease progression as shown by a decline in the range of motion of their arms (“reachable workspace” or RWS). From this placebo group, 36 individuals joined the open-label study and went on losmapimod for 48 weeks. This group, which had previously lost range of arm motion, demonstrated trends of slowing or stopping loss of muscle function, as measured by RWS. Participants who originally received losmapimod and remained on therapy (38 people), continued to show slowing or stopping of disease progression and demonstrated improvement in muscle function, as measured by RWS. (See graph below.)
Through the 96 weeks of the ReDUX4 and OLE study, losmapimod was generally safe and well tolerated by the participants. “No treatment-related serious adverse events (SAEs) or treatment-emergent adverse events (TEAEs) leading to study drug discontinuation were reported through 96 weeks of dosing,” Fulcrum noted.
“Every FSHD patient faces relentless and accumulating muscle and functional loss. These long-term data further demonstrate that losmapimod can provide meaningful benefits to patients living with this relentless, debilitating disease,” said Bryan Stuart, chief executive officer at Fulcrum. “The sustained ability to slow or halt the progression of FSHD over two years underscores the significance of our Phase 3 REACH trial and the potential of losmapimod to be the first approved treatment for FSHD.”
Fulcrum is currently investigating losmapimod in the ongoing Phase 3 REACH trial. For more information about the trial please visit https://clinicaltrials.gov/ct2/show/NCT05397470.


If a drug is approved for patients over 18, what will it mean for children?
Will more years of testing be needed?
This is my question as well. I have a 13 year old who will be approximately 15 when phase 3 ends. How old will FSHD patients have to be to receive the medication if it’s approved?
Ok so why not allow expanded access now for those most severely affected and do not qualify for the trial?
I agree with you wholeheartedly. I was told by my doctors at barnes jewish that the expanded access wasn’t yet ready because of “lack of resources” or “not enough of the drug” or whatever. If that’s the case why even have expanded access in the first place?????????
I agree. FSHD patients are declining every day. We need help now. Please make this drug available to patients as soon as possible.🙏
cool news
I am one the early participants on the initial trial and I feel that it the drug is at least slowing the progression. Time will tell as the participation increases in phase 3.
Good news
Ben FSH kas hastasıyım bu ilaç nezaman çıkacak
Is this trial closed for new participants? I was recently diagnosed with FSHD.
If not how do I find out about any new or upcoming trials?
Thanks
How old will patients need to be if the drug is approved?
If and when the drug is approved by the FDA, doctors will most likely be able to prescribe it to patients of any age. What is unknown is whether insurance companies will refuse to pay for treatment for certain individuals.
Hello,
Are these dates typically consistent? Conservative date? Realistic date? Padded date?:
ClinicalTrials.Gov – (FSHD) (Reach)
Actual Study Start Date: June 16, 2022
Estimated Primary Completion Date: March 2024
Estimated Study Completion Date: March 2024
Wo kann ich mich für die neue Losmapimod-Studie die demnächst in München starten soll bewerben?
Hallo Angelika,
geh bitte auf diese Seite: https://ichgcp.net/de/clinical-trials-registry/NCT05397470
ganz unten sind die Kontaktadressen von 3 Standorten in Deutschland.
Wie und wo könnte meine Ärztin Losmapimod bereits jetzt erhalten, denn bis es vielleicht 2026 auf dem Markt ist, wird meine FSHD so fortgeschritten sein, dass ich mich lieber vorher von dieser ach so schönen Welt verabschiede, denn in einem Rollstuhl zu sitzen, wäre keine Option für mich…