Patients are critical in helping to transform optimism into reality
by Ken Kahtava, Chief Business Officer, FSHD Society
Breakthroughs in FSHD research have identified the primary mechanism that causes FSHD. It is when a normally silenced gene, DUX4, gets “turned on.” When this happens, the gene causes toxic health issues in the body. The discovery of this mechanism has led many biopharmaceutical companies to actively work on DUX4-targeted therapies.
Research is quickly moving into human clinical trials. Fulcrum Therapeutics’ REACH trial is currently in Phase 3, and at least three more companies have reported plans to start their trials in 2023. Other companies are likely to launch clinical trials over the next few years as well.
There is genuine hope in the therapeutic development pipeline for people affected by FSHD.
Now for the challenging news
The FSHD Society, through meetings with industry executives and researchers, has identified several critical gaps that could slow the delivery of promising FSHD therapies, or even cause clinical trials to fail. We are working to close these gaps by expanding clinical trial readiness around the world.
This work is multifaceted and ranges from expanding the number of clinical trial sites around the world to building a global patient registry and better understanding the FSHD patient journey. It includes conducting research that builds evidence to address the socioeconomic burden of disease and providing data on fair pricing for drugs once they are approved.
All of these issues are critical to speeding the delivery of approved therapies to FSHD patients everywhere. We will break each problem down and share details of what the FSHD Society is doing to address it in future Advocate articles.
But there is one challenge that only patients can ultimately solve – volunteering for clinical trials.
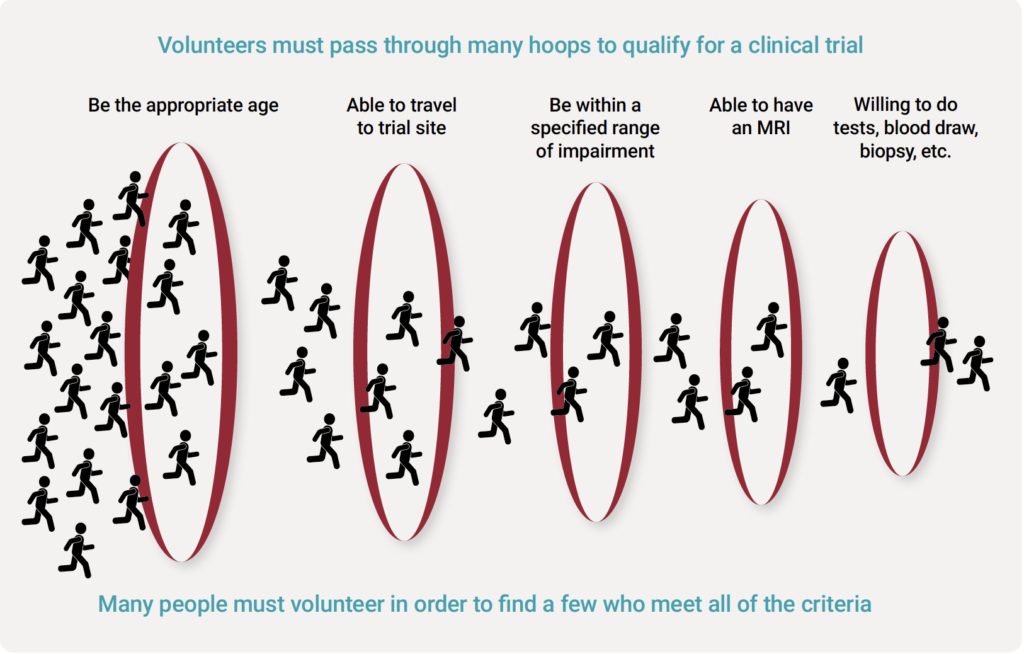
One of the major hurdles for successfully completing clinical trials is recruiting enough patients to participate in a reasonable period of time. This is exceptionally challenging for a rare disease like FSHD, compared to more common diseases.
For example, a clinical trial in diabetes would need to enroll only a tiny fraction of a percent of the roughly 37 million Americans affected by diabetes. Conversely, a FSHD trial may need to recruit as many as 1% to 2% of all people affected by FSHD in a geographic area. What’s more, biopharmaceutical companies must find and enroll patients in an acceptable period of time, sometimes only a few months.
A small biotech might need $100,000 a day to stay in operation, and if a trial takes too long to enroll enough patients, the company will be forced to shut it down so that they will not run out of the cash they need to survive.
Because of these challenges, the biopharmaceutical industry reports that more than 25% of rare disease trials fail. Considering the number of FSHD clinical trials on the near horizon, this is a real threat for FSHD.
Your involvement is essential
Finding enough willing individuals who meet the various criteria for current and future FSHD clinical trials is a tall task. The FSHD population is thinly dispersed around the world, with variable muscles affected at different stages of progression, and only a subset who will meet the criteria for a clinical trial.
While clinical trials offer no guarantees that a specific intervention will work, trials are the only way to get to treatments that do work. There is often confusion and uncertainty around deciding whether or not to participate in a clinical trial. We encourage you to go to our website to learn more about clinical trials and your opportunities to participate. Talk to your health care team and your family. By obtaining all the information you can, you can make an informed decision about whether clinical trial research is for you.
Keep an eye out for future issues of FSHD Advocate and follow the FSHD Society on our website and social media. We will be reporting on the many exciting initiatives coming in 2023. Your involvement is essential to meet the many clinical trial challenges. Together, we can ensure that FSHD clinical research advances robustly and ultimately delivers treatments and a cure for everyone living with FSHD.


Maybe the trials should broaden their requirements for range of impairment and age.
Sign me up for clinical trials, no spam or asking for donations email’s please!
The Society can’t sign you up for a trial. You will need to contact a trial site to ask about enrolling. Our emails to you will alert you to trials that are recruiting in your region. We don’t send spam, and you can opt out of fundraising emails.
I have tried to get on trials for many years and never hear anything back unfortunately
Same here. I have asked multiple companies to be part of their trials but never hear back. It seems there are gaps in the recruiting process from the company’s staff that should be addressed.
Dear sir/Madam
I am writing in regards to the participation in clinical trials, I live in the United Kingdom and have been constantly asking my health care professionals of when the trials are to,commence in the United Kingdom.
There will be two sites in the UK for the Losmapimod trial. They aren’t recruiting yet, but we recommend you contact them to indicate your interest.
University College of London Hospitals Not yet recruiting
London, United Kingdom, WC1N 3BG
Contact: Louise Germain 0203 108 6308 l.germain@nhs.net
Newcastle upon Tyne NHS Foundation Trust Not yet recruiting
Newcastle upon Tyne, United Kingdom, NE1 3BZ
Contact: Professor Jordi Diaz Manera 0191 241 8610 nmd.clinicaltrials@newcastle.ac.uk
I must reply that it is quite frustrating when I read a plea for more folks with FSHD to become involved in clinical trials, and to have been turned down for Fulcrum Phase 3 because I am 4 months over the age 65 cutoff, especially when the important screens are in physical condition, progression, ability and DNA. The age cutoff in this trial seems quite arbitruary.
That is a tough situation Mark. Two thoughts – Do you have family members who are affected with FSHD who are not yet engaged in the commmunity who might qualify for the REACH study? Have you considered getting involved in the MOVE study? I believe this natural history study doesn’t have age requirements and is another important study being done to characterize the progress of FSHD.
Best regards, I live in Colombia (South America). Is there a chance to participate in a trial?