Sincere thanks to our members who reviewed the performance of the FSH Society and helped us earn our fifth consecutive Top-rated Great Nonprofits badge! We work hard for all of… Read More »
We’ve earned our 2017 Great Nonprofits badge!
A new DUX4 mouse with muscle disease
The FSH muscular dystrophy scientific literature finally has a publication describing a genetic mouse model that develops skeletal muscle disease. This work comes via the laboratory of Michael Kyba, PhD,… Read More »
Testosterone and human growth hormone clinical trial for FSHD
UPDATED October 30, 2017 Researchers at the University of Rochester in New York are conducting a research study to learn more about a potential symptomatic therapy for FSHD. This study… Read More »
Carden Wyckoff elected to Board of Directors
Youngest member, with a passion for advocacy and fundraising The FSH Society is pleased to announce that Carden Wyckoff was elected to serve on the Board of Directors on September… Read More »
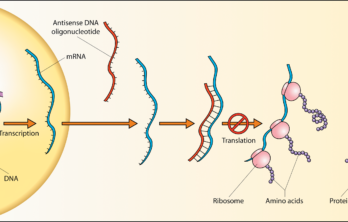
An FSHD Antisense Therapy Primer
Q&A With Dr. Yi-Wen Chen by JIM ALBERT, Eldersburg, Maryland Antisense therapy is a form of treatment for genetic disorders. In the past year antisense drugs have been approved by the… Read More »